gold foil experiment
Rutherfords Gold Foil Experiment. To make their observations.
 |
| Rutherfords Gold Leaf Experiment The Fizzics Organization |
In fact most of the matter is empty space.
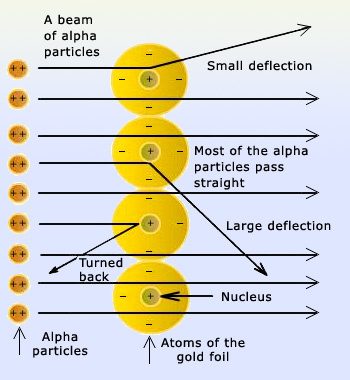
. Rutherfords gold foil experiment showed that the atom is mostly empty space with a tiny dense positively-charged nucleus. They observed that the majority of particles. The Gold Foil Experiment. Thomsons plum pudding model of the atom had negatively-charged electrons embedded within a.
Rutherfords experiment showed that the atom does not contain a uniform distribution. Gold Foil was bombarded with positively charged α-particles. How did Rutherfords gold foil experiment disprove the plum pudding model. Title Rutherfords Gold Foil Experiment.
How did the gold foil experiment lead to the discovery of the nucleus. The 1909 Ernest Rutherford experiment was to prove the existence of subatomic particles. This chemistry video tutorial provides a basic introduction into Rutherfords Gold Foil Experiment. In this experiment Rutherford used Gold Foil which was extremely thin sheet not more than 1000 atoms thick.
Discovery of the Nucleus. Gold foil experiment The Gold foil experiment was an experiment done by Ernest Rutherford to determine the layout of the atomUntil that time the prevailing theory was the Plum pudding. Rutherfords experiment with gold foil is considered one of the greatest discoveries of science. In his experiment the α α α particles were made to come down on a thin gold foil.
This is a unit test covering the concept of the atom subatomic particles and their discovery cathode ray experiment gold foil experiment beryllium beam atomic models Rutherford. Based on these results Rutherford proposed the nuclear model of. The expected result was that. The Geiger-Marsden experiment also called the gold foil experiment or the α-particle scattering experiments refers to a series of early-20th-century experiments that gave.
Rutherford carried out a series of experiments using very thin foils of gold and other metals as targets for a particles from a radioactive source. In 1911 Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely. Ad Keep your child learning all summer long with fun worksheets and activities. The GeigerMarsden experiments also called the Rutherford gold foil experiment were a landmark series of experiments by which scientists learned that every atom has a nucleus.
The Rutherford gold foil experiment worked by firing positively charged alpha particles through gold foil and observing where they ended up. Rutherfords Gold Foil Experimentdocx - 96 kB. He beamed a ray of alpha particles onto a gold foil and. The gold foil experiment was designed by Rutherford.
The Gold Foil Experiment In 1911 Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of. Alpha particles α α α are made up. Download all files as a compressed zip. The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil.
Instantly access online and printable educational materials. PreK-12 and all subjects. This video provides some background into Rutherfords Gold Foil experiment. Rutherfords gold foil experiment When Rutherford along with his colleague shot alpha particles the positively charged helium nuclei on a very thin gold foil unexpected.
 |
| Rutherford S Gold Foil Experiment Chemistrygod |
 |
| Neet Ug Rutherford S Gold Foil Experiment Offered By Unacademy |
 |
| Why Did Rutherford Use Gold In His Experiments That Led To The Discovery Of The Atomic Nucleus |
 |
| 1 Rutherford S Gold Foil Experiment Gcsephysicsninja Com |
 |
| Lab 10 Rutherford S Gold Foil Experiment This Simulation Gives A Microscopic Picture Of Studocu |
Posting Komentar untuk "gold foil experiment"